Background:
Newly diagnosed chronic lymphocytic leukemia (CLL) patients are monitored regularly for disease progression to delay the impact of treatments on the patients' quality of life (QoL). However, studies have reported mixed results on the QoL between active surveillance patients compared to active treatment patients. Herein we performed a cross-sectional study to examine the impact of active surveillance and active treatment on health-related QoL in CLL patients.
Methods:
We included 210 CLL patients in Israel, who completed an online survey with demographic and disease characteristics questions; the Functional Assessment of Cancer Therapy-General (FACT-G), a general health scale including the following subscales: physical well-being, social well-being, emotional well-being, and functional well-being; symptoms and concerns related to leukemia (FACT-LeuS); and FACT-Leukemia (FACT-LEU), a total score derived from the FACT-G and FACT-LeuS scores. Among participants, 63 were in active surveillance, 115 in active treatment (71 in BTKi based treatment and 44 in venetoclax based treatment), and 32 completed their treatments. We compared CLL patients in the surveillance group versus the active treatment group using the Student's T test. Analyses were also stratified by sex. In addition, we compared CLL patients in four groups: active surveillance, treated with BTKi, treated with Venetoclax based treatments, and completed treatments, using ANOVA, and the Hochberg's adjusted P value. For all analyses we estimated the effect size between each two groups using the Cohen's d measure and 95% confidence intervals (CI).
Results:
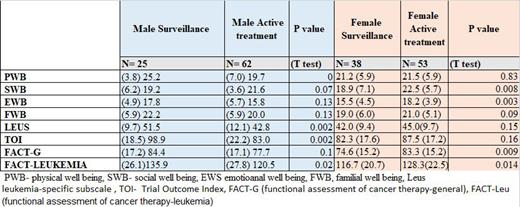
Overall, the mean age of participants was 64 (SD=10) with 40% males in the active surveillance group, and mean age of 67 (SD=10) with 54% males in the active treatment group. Patients in the surveillance group reported lower QoL scores compared to the active treatment group in the social/family well-being subscale (19.0±6.7 vs 22.1±4.7, P=0.002) with a medium effect size [-0.56, CI:(-0.87,-0.25)], however, in the physical well-being subscale they reported a higher QoL scores (22.8±5.5 vs 20.5±6.3, P=0.017), with a small effect size [0.38, CI:(0.07,0.69)]. Among males, patients in the surveillance group reported higher QoL scores compared to the active treatment group in the physical well-being subscale (25.2±3.8 vs 19.7±7.0, P=0.0), with a high effect size [0.87, CI:(0.38,1.35)], FACT-LeuS score (51.5±9.7 vs 42.8±12.1, P=0.002), with a medium effect size [0.76, CI:(0.28-1.23)], and the FACT-LEU score (135.9±26.1 vs 120.5±27.8, P=0.02), with a medium Effect size [0.56, CI:(0.09-1.03)]. Among females, patients in the surveillance group reported lower Qol scores compared to active surveillance in the social/family well-being subscale (18.9±7.1 vs 22.5±5.7, P=0.008), with a medium effect size [-0.58, CI):-1.0,-0.15)]; emotional well-being subscale (15.5±4.5 vs 18.2±3.9, P=0.003), with a medium effect size [-0.66, CI):-1.09,-0.23)]; FACT-G (74.6±15.2 vs 83.3±15.2, P=0.009), with a medium effect size [-0.58, CI):-1.0,-0.14)]; and FACT-LEU (116.7±20.7 vs 128.3±22.5, P=0.014), with a medium effect size [-0.53, CI):-0.96,-0.11)]. When comparing the four groups of treatments, we found significant differences in the physical well-being subscale (P=0.029), which was driven by the lower score in the Venetoclax based treatment group compared to the surveillance group [19.6±7.3 vs 22.8±5.5, medium effect size 0.49, CI:(0.10,0.88)]; and social/family well-being subscale (P=0.005), which was driven by the higher score in the BTKi based treatment group compared to the surveillance group [22.3±4.3 vs 19.0±6.7, medium effect size -0.53, CI:(0.87,-0.19)].
Conclusions:
In general, males in active surveillance reported better health-related QoL than those in active treatment, while females in active treatment reported better health-related QoL than those in active surveillance. Interventions for improving QoL in CLL patients should include gender-based perspectives.
Disclosures
Tadmor:janssen,: Research Funding; Abbvie, ROCHE, Janssen, AstraZeneca, takeda: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal